The NEIF Argon Laboratory has been working for the past few years on developing dating of low temperature, supergene (and hypogene) minerals such as alunite, jarosite and cryptomelane.
These minerals have significant potassium content making them amenable to 40Ar/39Ar dating.
Jarosite : KFe3(SO4)2(OH)6 (11.37 wt.% K2O)
Alunite : KAl3(SO4)2(OH)6 (9.40 wt.% K2O)
Cryptomelane : K(Mn4+,Mn2+)8O16 (6.41 % K2O)

Weathering of Mesozoic sedimentary deposits in Utah, US.
They form in hydrothermal systems or weathering horizons via the interaction of hydrous fluids and host rocks, thus providing an important way to constrain the ages of these processes.
We have three facets to our interest in these minerals.
Economic : Dating of these minerals has been used to constrain the formation age of some of the world’s largest economic deposits (e.g, bauxites, Vasconcelos 1999). Our hope is to expand our research into critical metals deposits and contribute to a green energy and circular economy future.
Climate : We are interested in weathering processes from a terrestrial standpoint as these minerals can form in response to climate change thus providing an on-shore climate archive to complement other climate records, reaching back into the Cenozoic.
Planetary : Recognition of jarosite by three Mars rovers has generated significant interest in the scientific community as jarosite requires the presence of water to form. Being able to date jarosite on Mars may allow us to constrain when liquid water was available on the Martian surface and speculate on the potential for life supporting conditions.
Our initial forays into this field of dating were with alunite and jarosite, with some early results presented below.
Technical Aspects – Sample preparation
Preparation of alunite and jarosite requires selecting the purest material available in the field. Texturally we are looking for dense, massive samples, preferably free from any host rock minerals. It is best practice to characterise your samples with SEM imaging and XRD analysis to constrain textures, crystallinity and potential contamination.
As these minerals form via replacement or precipitate from solutions derived from weathering, they often are intimately intergrown with pre-existing silicate or sulfide phases. This poses a challenge for sample preparation but can be overcome with various preparation techniques such as SELFrag, magnetic separation, heavy liquid separation and acid leaching for example.

Sample preparation and laser fusion analysis of our first alunite samples.
Unfused and fused alunite from Rodalquilar, Spain. Results (in triplicate) below. The data show good agreement, but clearly highlight the air-rich nature of such materials that, despite high K content, limit precision. Finding ways of reducing air-contamination is one of our technical goals.
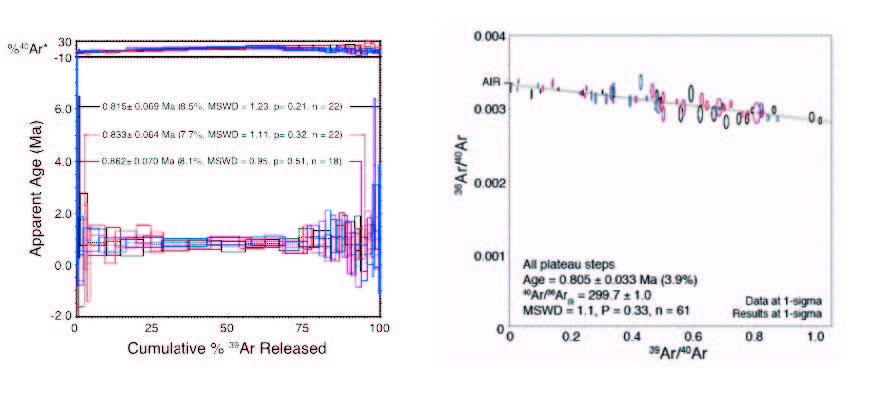
Does leaching help? Opinions vary. The drive to use leaching is to avoid the potential for inherited argon from silicate contamination (clays, felspars, micas, etc.) (Samuels-Crow et al. 2012). However, other workers avoid acid leaching, preferring to select high purity materials from the outset. However, work we’ve done to directly investigate the effects of leaching suggest that leaching helps to reduce the amount of air in a sample. We speculate that this results from the preferential removal via leaching of fine-scale or poorly crystalline alunite, leaving behind coarser alunite grains with reduced surface area or reduced interstitial spaces that host air. We hope to do further work to confirm this with parallel SEM work.
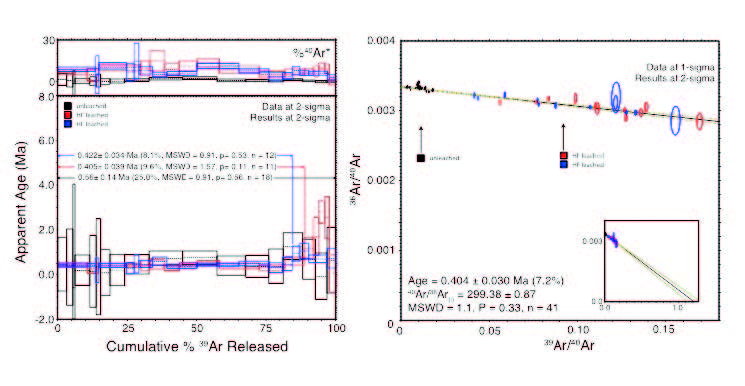
Alunite from Greece. Note the separation in the isochron diagram between leached and unleached material. Leaching reduces atmospheric contamination, improving precision by at least a factor of 3 on plateau ages.
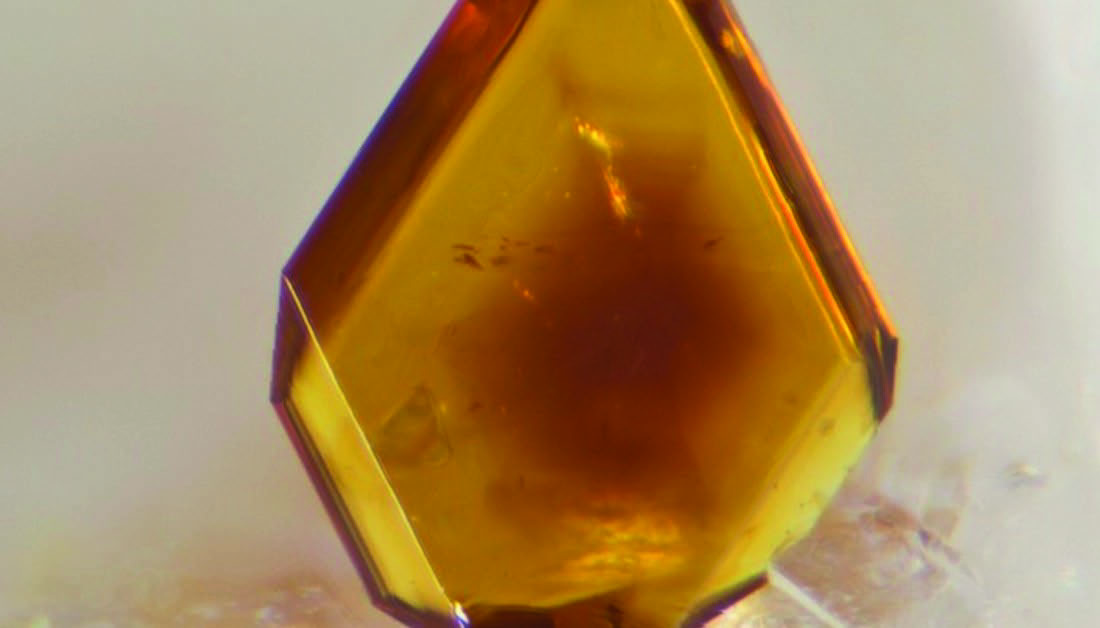
Jarosite from the type locality, Mina La Estrella, Barranco Jaroso, Almería, Andalucia, España (courtesy of Mindat.org, no copyright listed).
References :
Samuels-Crow, K., Lueth, V., Peters, L., & McIntosh, W. (2012). 40Ar/39Ar geochronology of jarosite: The effectiveness of HF in removing silicate contaminants. Chemical Geology, 314-317, 23-32.
Vasconcelos, P. (1999). K-Ar and 40Ar/39Ar Geochronology of Weathering Processes. Annu. Rev. Earth Planet. Sci.(27), 183-229.
